Point-Of-Care Verification
HIPA-TRAKKER™ RFID-BLOOD SUPPLY PEDIGREE MANAGEMENT MODULE
(Solution based on granted patents, allowed and pending US patents)
- Access and Identification function.
- RFID badge of all individuals involved in screening and drawing blood.
- Registration data of donor’s individual information is entered only after verifying permission level and correlating presenting worker’s RFID badge.
- All donors are given an RFID wristband during registration.
- The personal ID of the registration staff is preferably included.
- Label printing and encoding can only be accessed and performed by workers with authorized RFID badges.
- Donor Identification and Blood Sample Matching Function.
- Label for blood sample is printed and encoded by scanning the RFID wristband of the donor.
- Unitary RFID label is printed and encoded at the same time.
- RFID tag is behind the human readable label with barcode.
- The ID of the staff involved in the process is preferably included in the RFID label.
- Actual drawn blood container is also labeled with integrated RFID/barcode/human readable label using data directly obtained from scanning the donor badge.
- Blood Consolidation Matching Function.
- Each blood sample is first scanned before consolidation.
- Check is made against blood type, etc.
- The data of the blood sample is recorded and included in the consolidated RFID label.
- The data preferably includes the person(s) performing such operation.
- This enables tracing of blood from each individual donor.
- Subsequent Blood Preparation Function.
- All operations must be first accessed with proper worker ID.
- The data of the blood sample is maintained through to the resulting blood ingredient derived from the original blood samples.
- All subsequent RFID labels include the donors’ data along with the workers handling them throughout the process.
- Blood Usage Matching Function. The staff responsible for the blood usage must be first be scanned.
- The recipient wristband must be then scanned.
- The blood sample to be used must be scanned to match that of the recipient blood type.
- All other relevant data must also be confirmed, such as expiration date, etc.
- The authorization may include biometric signature or other e-prescription as required by the local management.
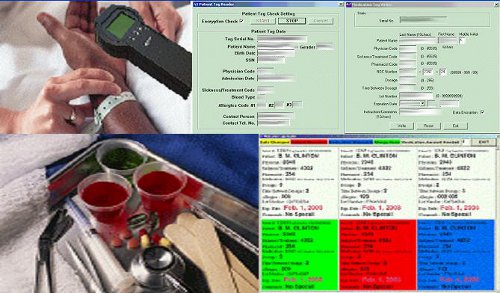
HIPA-TRAKKER Positive Patient Medication Matching Module (5 Rights Module)


